Creat UDI CODE For Dental Supplies:How to make it on your package?
- Regaridng to UDI code, we’d like to give you a brief introduction:
- 1). UDI is request by FDA and it is a unique device identification system designed to adequately identify devices through distribution and use.
- 2). Usually speaking, all the packagings will need to have this UDI code, for example, the boxes, master carton (cases).
- 3). A UDI is a unique numeric or alphanumeric code that consists of two parts:
- · A device identifier (DI), a mandatory, fixed portion of a UDI that identifies the labeler and the specific version or model of a device,it should be provide by importers(our customers).
- · A production identifier (PI), a conditional, variable portion of a UDI that identifies one or more of the following when included on the label of a device:
- the lot or batch number within which a device was manufactured;
- the serial number of a specific device;
- the expiration date of a specific device;
- the date a specific device was manufactured;
- distinct identification code
- 4).Now all of our USA customers has use this UDI code for Class II self sterilization pouches, Class I dental supplies and Health Canada is also in the process of implementing this system, the clients from Canada need to be ahead of the regulation in Canada as well.
- Steps for beginner to creat UDI code for dental products packages:
- 1). If you need to follow the FDA Guides or follow the US maket regulations, you need find out the UI or DI code of the products that you import. (Normally your FDA agent could help you to get the UI or DI code.)
- 2). All UDIs are to be issued under a system operated by an FDA-accredited issuing agency. If you need to know more about that, you can visit below website:
- 3). After you get the UI or DI number, then you can create the UDI barcode according to the regulation.
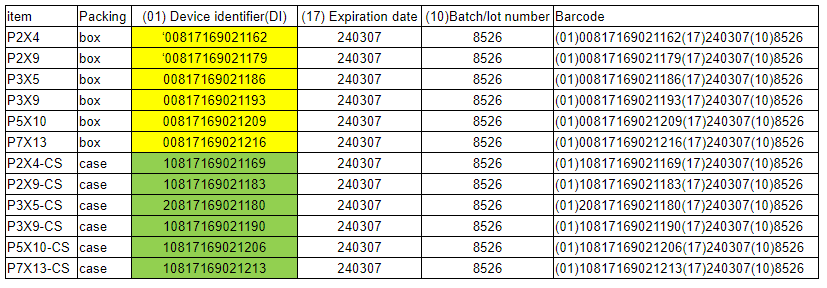
- 4). Some samples of our current customer’s UDI code.
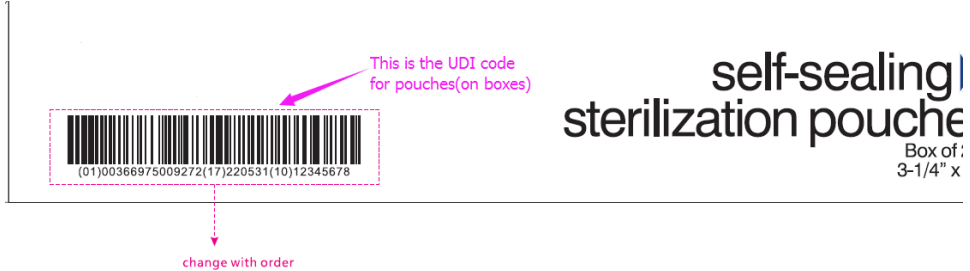
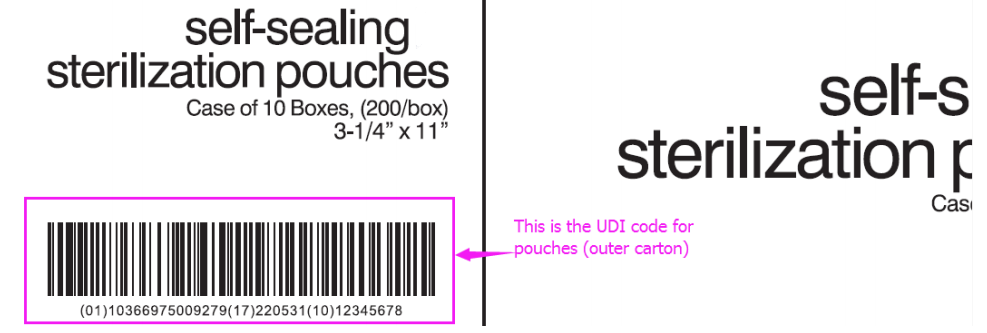
- Different customer has different requirement on the format of the UDI code rules, for example:(01) Device identifier+(17)Expiration date+(10)Batch/lot number.
- (01) is your UI or DI code, which is usually provided by customers.
- (17) is the Expiration date, which is provide by manufactures according to the prodcution date.
- (10) is the lot no, we usually use customer’s PO NO. as the lot no.

cialis 5mg best price
thank you